
A Complete Account of EMA Approvals in 2024
Shots:
-
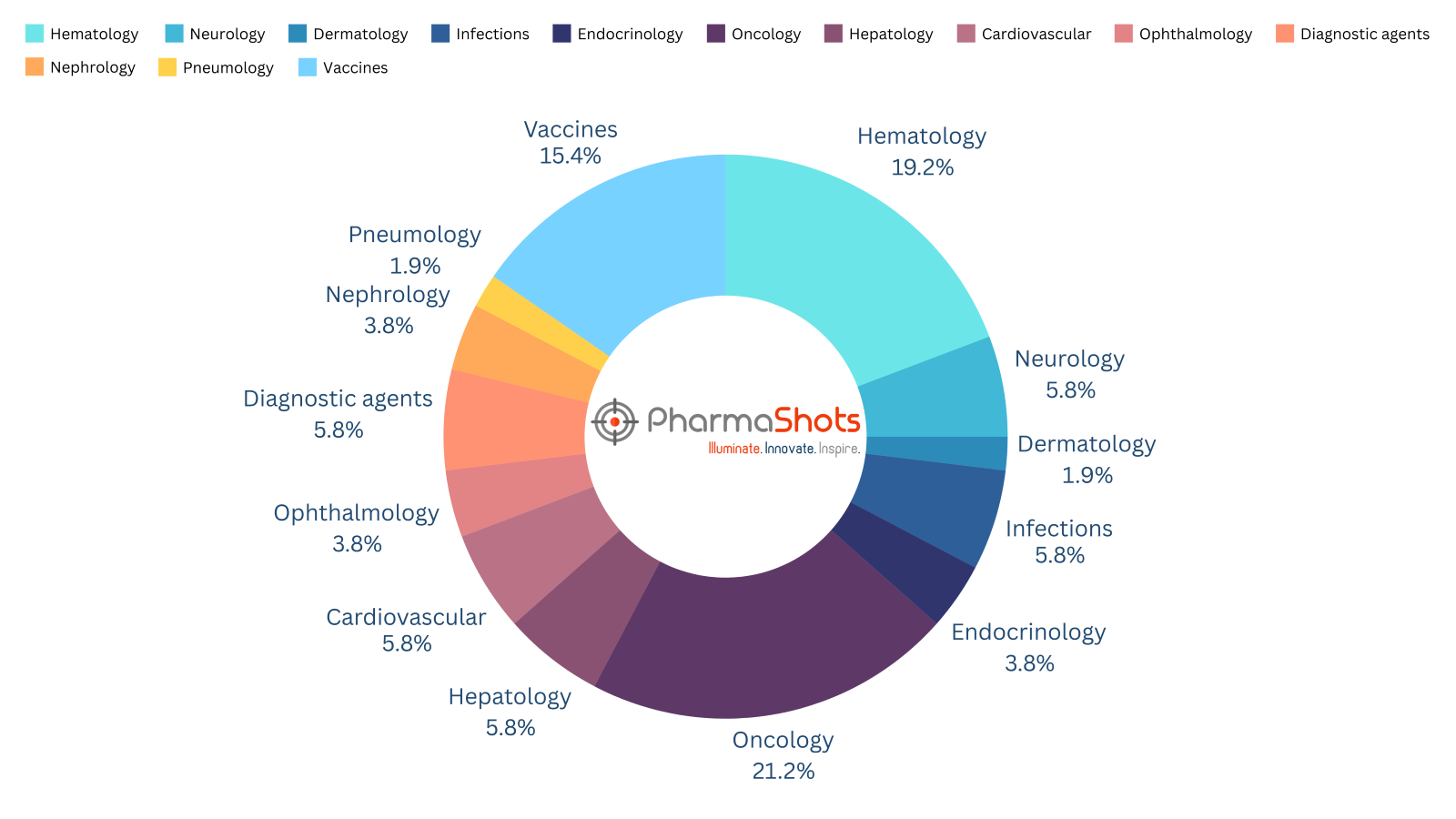
In 2024, EMA approved around 53 drugs in various therapy areas, ranging from cardiology, oncology, hematology, neurology, and dermatology to infectious diseases, vaccines, diagnostic agents, pneumology, nephrology, hepatology, ophthalmology, and endocrinology
-
PharmaShots, in an illuminating report, brings a condensed analysis of the approved drugs with the most explored areas being Oncology, Hematology, Neurology, & Dermatology
-
For the complete report with analysis, reach out to us at connect@pharmashots.com
Unveiling a second package of delights that is sure to take you down memory lane with PharmaShots EMA's new drug approvals in 2024. The report outlines the therapy areas of approvals, the company involved, and the approval date.
Oncology
2024 witnessed 11 noteworthy approvals. J&J’s Balversa is approved as a monotherapy for the treatment of adult patients with unresectable or metastatic urothelial carcinoma (mUC), harbouring susceptible FGFR3 genetic alterations who have previously received at least one line of therapy containing a PD-1 or PD-L1 inhibitor in the unresectable or metastatic treatment setting
AstraZeneca’s Truqap is approved by EMA for patients with estrogen receptor (ER)-positive, HER2‑negative locally advanced or metastatic breast cancer with one or more PIK3CA, AKT1, or PTEN-alterations following recurrence or progression on or after an endocrine-based regimen.
Takeda’s Fruzaqla is used in adults to treat metastatic colorectal cancer (mCRC) who have been previously treated with available standard therapies, including fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapies, anti-VEGF agents, and anti-EGFR agents, and who have progressed on or are intolerant to treatment with either trifluridine-tipiracil or regorafenib
Novartis’s Spexotras is a small molecule to treat children age≥1 yrs. with glioma
Hematology
In 2024, EMA approved 10 drugs in Hematology domains. Pfizer’s Hympavzi is approved by EMA to prevent bleeding episodes in patients age≥12 yrs. weighing at least 35 kg with severe hemophilia A or B. Roche’s Piasky treat adults and children over 12 years of age weighing 40 kg or more who have paroxysmal nocturnal hemoglobinuria (PNH)
Sobi and Sanofi’s Altuvoct is used to prevent and treat bleeding in adults and children with hemophilia A. Vertex Pharmaceuticals’ Casgevy is a gene therapy used to treat blood disorders known as beta thalassemia and sickle cell disease in patients age≥12 yrs
The report only covers the highlights of Oncology and Hematology. For a complete report, reach out to us at connect@pharmashots.com
Related Post: A Complete Account of EMA Approvals in 2023
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














